Navigation auf uzh.ch
Navigation auf uzh.ch
|
|
|
PD Paolo Cinelli, PhD
|
Prof. Hans-Christoph Pape, MD
|
The research focus of the Department of Trauma is the improvement of the treatment of severely injured patients. We are interested in all aspects of research that can improve treatment of severely injured patients at basic, translational and clinical levels. Our main interests are the study of the pathophysiology of trauma, the development of regenerative approaches for improving bone healing and the impact and treatment of fractures in older patients. To approach these clinically relevant challenges we have built a team of surgeon scientists and basic scientists that closely work together.
Traumatic injuries induce a complex host response that disrupts immune system homeostasis and trigger a systemic inflammatory response that predisposes patients to opportunistic infections and inflammatory complications leading to secondary complications, such as nosocomial infections, sepsis or multi-organ failure. Our studies aim at the identification of mechanisms linked to complicated courses after severe trauma by a systems biology approach combining clinical data and omics (transcriptomics, proteomics and metabolomics) approach. This approach is useful to improve the prognostic performance and individual risk stratification in trauma patients. In parallel to clinical studies on patients we have established a porcine polytrauma model and are analyzing locally, at the site of injury, and systemically how inflammation and immune response are initiated and how they are regulated (Figure 1). This standardized model allows to study the timely changes in local and systemic inflammation following multiple injuries. The combination of clinical and translational studies allows further dissecting of the molecular mechanisms underlying these physiological changes.
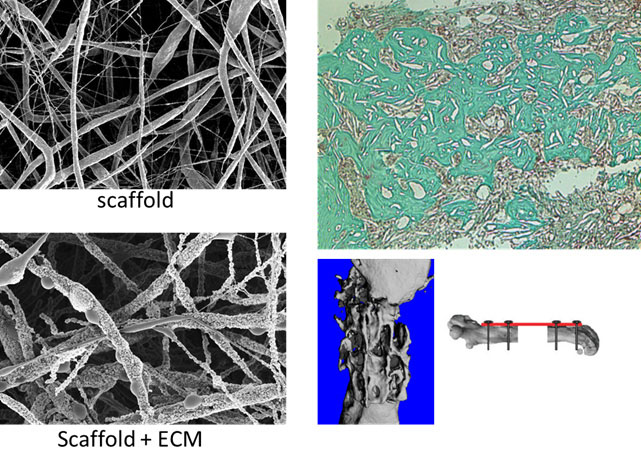
The capacity of bone to regenerate is of outmost importance in the case of injury. Regeneration consists of a well-orchestrated series of biological events that comprise bone induction and conduction, involving a number of cell types and intracellular and extracellular molecular signaling pathways, with a definable temporal and spatial sequence, with the final goal to optimize skeletal repair and restore skeletal function. The process of fracture healing involves many events including the signaling, recruitment and differentiation of skeletal stem cells during the early phase; formation of a hard callus and extracellular matrix, angiogenesis and revascularization during the mid-phase; and finally callus remodeling at the late phase of fracture healing. Unlike in other tissues, the majority of bony injuries (fractures) heal without the formation of scar tissue, and bone is regenerated with its pre-existing properties largely restored, and with the newly formed bone being eventually indistinguishable from the adjacent uninjured bone. Despite the fine degree of orchestration during fracture healing, the process may be impaired. Currently, 10–15% of the fractures that occur annually result in poor or unresolved healing, so called non-unions or critical size defects. These fractures which cannot heal completely from alone over a long period of time represent a major clinical orthopedic surgery. The gold standard treatment is the use of autogenous bone grafts in combination with alloplastic material. Usually the iliac crest is used as a donor site for bone harvesting. Nevertheless, this procedure has some drawbacks for clinical applications, such as limited availability of bone graft, morbidity, and donor site pain. Alternatively, biocompatible materials can be used but unfortunately, to date no single synthetic material offers all the benefits of the patient’s own bone. Tissue engineering represents a very promising technique, which combines the use of stem cells with scaffold of synthetic or natural biomaterial together with molecular signals, such as growth or differentiating factors. Mesenchymal stem cells (MSCs) represent a good source of regeneration-competent cells. They can be isolated from a variety of tissues and are able to differentiate under the right culture conditions, into osteoblasts, chondrocytes, and adipocytes. The major problem with the use of MSCs isolated from bone marrow or fat tissue is that the isolated cells contain heterogeneous populations of stem and progenitor cells. Thus, for the clinical use of MSCs for regeneration purposes, it is urgently needed a better characterization of the cells and a standardization of the isolation and culture protocols. In our studies we test the possibility to enrich defined subpopulations of stem/progenitor cells for direct therapeutic application without requiring an in vitro expansion. The most promising enriched stem cells populations are tested for their regeneration capacity in mouse models. For the identification of new cell subpopulations we employ modern technologies like Cytometry by time-of-flight (CyTOF) allowing the real-time analysis of single cells in complex populations. Single cells, labelled with stable heavy metal isotopes are analyzed by a combination of classical flow cytometry and mass spectrometry analysis. We are also developing new scaffolds for bone regeneration by combining synthetically produced scaffolds with extracellular matrix (ECM) produced by stem cells (collaboration with Prof. Wendelin Stark and Olivier Gröninger, ETH). Cells are first seeded on electrospun scaffolds. After cultivation, the cells deposit on the scaffold surface ECM components and hydroxyapatite. In further step the scaffolds are being decellularized thereby eliminating the cells from the scaffold surface but retaining the ECM components and hydroxyapatite. The scaffold can be then stored and used when needed. This approach allows the preparation of off-the-shelf bone graft substitutes with low risks for rejection promoting constructive remodeling of bone tissue and eliciting various biologic responses including angiogenesis and chemotaxis of bone forming cells. We finally test our scaffolds in a mouse model for critical size bone injury.
Bone is a rigid organ that provides support and physical protection to various vital organs of the body and is permanently in a dynamic balance, a process called remodeling, which allows a constant regeneration of bones (e.g. in the adult human body, the entire skeleton is renewed every 7 years). The tightly regulated processes responsible for continuous bone remodeling involve a complex coordination of multiple bone marrow cell types: bone formation by osteoblasts and resorption by osteoclasts. An important clinical aspect of bone remodeling is the imbalance between bone formation and resorption, which results in various diseases, such as osteopetrosis, osteopenia, and osteoporosis
The population worldwide is ageing and life expectance is steadily increasing. The decrease in bone density and quality in osteoporotic patients leads often to fractures often as consequence of a fall from a standing height. Osteoporotic fractures are associated with high rates of morbidity and mortality and the overall cost of treatment is very high. The role of trauma surgery in older patients is therefore of great importance. The main goal of treatment is to provide stable fixation that allows early weight bearing and mobilization. Our research focuses on one side in optimizing the surgical procedures by assessing through biomechanical testing the stability of different osteosynthesis devices. On the other side we aim at studying the cellular events underlying the development of osteoporosis. A current hypothesis is that a decrease in the number and function of bone and bone marrow derived MSCs is responsible of age-related bone loss. In a current clinical study, we are making use of our newly developed cytometry by time-of-flight technology to monitor at single cell level the changes occurring in MSCs isolated from osteoporotic bone upon fracture.