Cardiac Surgery Research
Head
|
|
|
Prof. Dr. h.c. Omer Dzemali
|
PD Héctor Rodriguez Cetina Biefer
|
Organization
Novel devices: Interventional treatment of acute type-a dissection
M. Hofmann, M. Schmiady, G. Puippe (USZ, Interventional Radiology) P. Vogt, R. Bauernschmitt
Acute type-A dissection of the aorta is one of the most critical conditions in cardiovascular medicine, usually leading to immediate emergent surgery. Despite all improvements in surgical techniques, implant material and cardiopulmonary bypass, mortality and morbidity after surgery is high and did not change during the last decades.
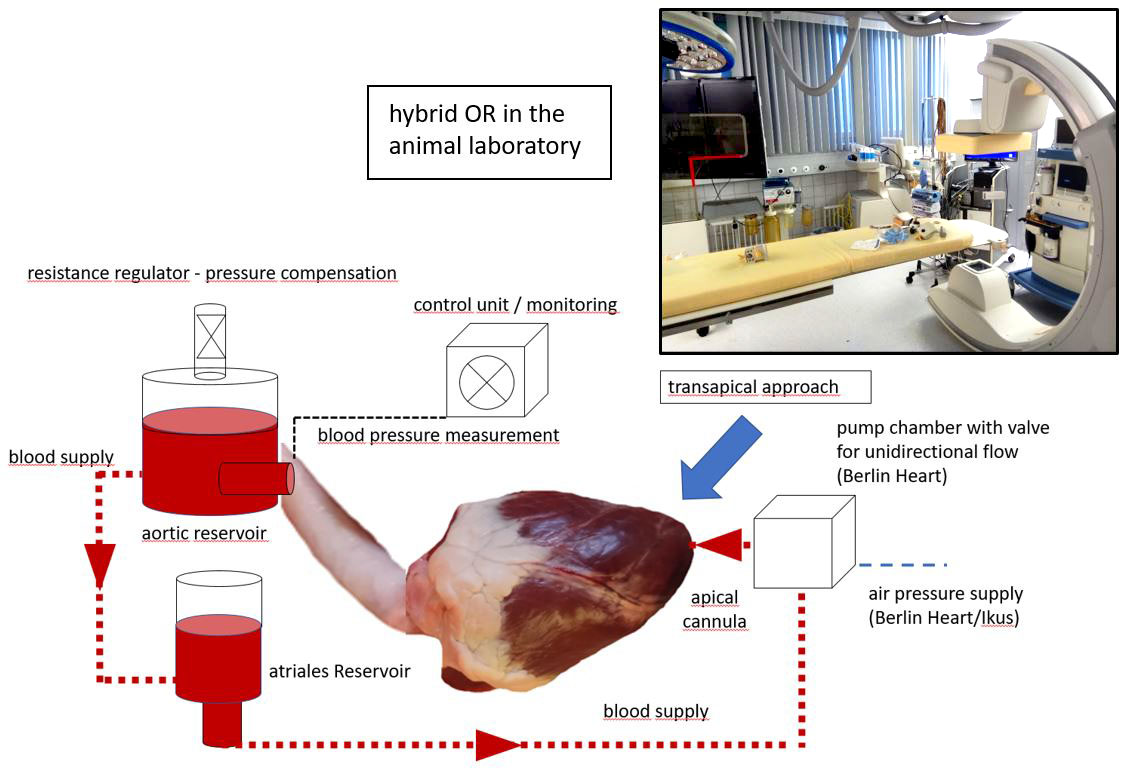
Thus, the need for an interventional device has been claimed. Due to the frequently accompanying aortic valve insufficiency, the variable take-off of the coronary arteries and the anatomical variations of the ascending aorta and the location of the intimal tear several challenges have to be met to develop an immediately available graft meeting the needs of these patients in emergency conditions. To test various prototypes, a mock-circulation with the possibility to include biological tissues has already been realized.
Autonomous ICU by fuzzy-logic and artificial intelligence (cooperation with Chair for robotics and Embedded systems, TU Munich)
R. Bauernschmitt, A. Knoll (TU Munich)
At present, ICU-treatment depends on manual control performed by physicians and nurses. Many of these tasks can be transformed to a fuzzy-logic-based, autonomous and intelligent closed-loop control system. This may lead to a massive decrease oft he human workload. Control of inotropic medication and volume substitution can be transferred from former work of our group. Also automatic, intelligent control of extracorporeal pumps and oxygenatores has already been realized and published during earlier cooperrations. An addition, control of mechanical ventilators is the goal of the present project, which shall be achieved by a combination of a fuzzy-logic rule network together with machine learning through artificial intelligence.
The aim of the project is to improve proactive, individualized therapy for the patient, but also to unburden physicians and nurses to meet the demands of further pandemic situations.
Surface modification of biological valves with femtolaser treatment (cooperation with NoviNano-institute, Lviv, Ukraine)
P. Vogt, R. Bauernschmitt, I. Gnilitskyi, (Lviv, Ukraine)
While surgical repair is possible only in a minority of cases, implantation of heart valve prostheses is the only causal therapy in patients with advanced heart valve disease. Even state-of-the-art prostheses have a variety of shortcomings like thrombogenity (formation of blood clots on their surface), suboptimal hemodynamics and limited durability requiring additional operations or catheter interventions. The new generation of valve prostheses is supposed to be produced from biocompatible synthetic tissue, that, however, is treated by means of chemical and morphological modification of the surface towards a total blood- and cell repellent structure, thus preventing both blood clot formation and bacterial infection. The first step will be Developing and optimization of bloodphobic functional nano-microstructures on the surfaces of cardiac valves and stents by using femtosecond laser pulses.
- design bloodphobic laser-induced nano-micropatterning on metallic cardiac valves and stents. All experiment will be performed by using femtosecond laser system from Light Conversion “Pharos”.
- Laser-induced nano-microstructures will be optimized by means of tuning laser parameters (wavelengths, pulse duration, power). The laser parameters will be optimized to obtain high production of treated samples.
- The bloodphobicity of the samples will be tested by using tensiometer “Biolin Scientific”
- Subsequent small and large animal testing.
Cardiac surgery and autonomous nervous system: CRC after heart surgery (cooperation with Humboldt-University, Berlin)
R. Bauernschmitt, N. Wessel (HU Berlin)
The clinically available procedures for assessing the health and the prognosis of a patient during heart surgery are still too imprecise today and are only partially suitable for prognostic applications. The aim of the project is to characterise the health condition in the perioperative setting of cardiac surgery by the analysis of cardiorespiratory coupling (CRC). CRC investigates the mutual influence of cardiac and respiratory oscillations in their onsets, an increased CRC indicates an increased sympathetic tone. Preoperatively increased CRC values may be predictors for operation complications; a postoperatively increased CRC will show the stress due to the surgery but also inflammation.
The groups of the applicants already developed some methods for the detection and quantification of CRC, the pathophysiology however still is unknown. Therefore, different methods based on synchronisation and coordination, in time and phase space, will be developed for an advanced data analysis. Moreover, sophisticated models of cardiorespiratory dynamics will be developed to bring more insights into the physiology of this phenomenon.
The information obtained from CRC analysis is to be used in the future for preventive treatment, not least for the determination of the optimal time for treatment to prevent complications. In the context of a clinical study, the project will demonstrate for the first time, using methods of non-linear dynamics and biological physics, that the monitoring of the individual patients risk is possible and the basis for improved risk stratification during the perioperative setting of cardiac surgery.
MR-Evaluation of the effects of ECC on cerebral perfusion
M. Hofmann, M. Schmiady
The «Research Group Heart and Brain» of the Children– Hospital in Zurich works on brain damages caused by open heart surgery with heart-lung machine in children with congenital heart disease.The main focus is on neuropsychological and motor development. Optimizing the regimen of cardiopulmonary bypass in neonates has extensively been worked on, however, on-line-monitoring of cerebral damage by magnetic resonance imaging was not possible so far due to the incompatibility of both machines.
Thus, the goals of this project are the following:
- Development of an MRI-compatible heart-lung-machine
- Analysis of contemporary regimens of extracorporeal circulation and the detrimental effects on the central nervous system in a small animal experiment
- Optimizing these parameters and on-line control of their effects by imaging
- Proof of concept has already been successfully finished.
Cardioplexol: a new form of cardioplegic solution on its way to clinical use
Th. Carrel
A Multi-Center, Open Label, Single Group, Observational Study to Investigate the Effects of Training on the administration of Cardioplexol
In a recently investigated RCT, Cardioplexol showed noninferiority when compared to Buckberg blood cardioplegic solution. However, Cardioplexol was not administered as specified in the manufacturers protocol in 11.8% of the cases. This could indicate, that a training program may reduce the risk of incorrect administration.Primary and secondary Objectives: To explore the effects of a training program on the rate of correct application of Cardioplexol and to explore the effects of Cardioplexol on myocardial protection during ischemic period in on-pump cardiac surgery, and to evaluate the safety and tolerability of Cardioplexol. A multi-center, observational study was designed to evaluate the effects of a preparation and administration training program to cardiotechnicians and cardiac surgeons inexperienced in the use of Cardioplexol. The training program consisted of a theoretical and one practical section. A short exam was taken by all participants in order to make sure that all aspects of Cardioplexol- Administration were fully understood. Each surgeon’s first two surgeries with Cardioplexol were performed in the presence of a coach. Authorization for further use of Cardioplexol was only given when approved by the coach. After successful completion of the training program, each surgeon’s next consecutive 4 cases were performed without the presence of the coach. Parameters regarding the primary efficacy endpoint were collected during the surgical procedure, patients were evaluated with respect to safety secondary endpoints from beginning of surgery up to 30 days after surgery. Male or female patients between 18 and 80 years of age requiring primary elective CABG or cardiac valve repair/ replacement with an LVEV > 30% were enrolled in the study. A total of 25 surgeons were planned to be trained, accounting for a minimum of 150 patients to be operated in the study. Our study-center enrolled 5 active surgeons not familiar with the use of Cardioplexol. All surgeons successfully completed training and had two coached operations followed by 4 operations without further observation by the study designers/ Coaches. Data collection was completed in time. Study-results are not available yet.
Figure 2: Schematic drawing of the artificial circuit giving us the possibility to examine different designs of TAAD-stents without sacrificing living animals
Collaborations
- Dept. of Veterinary Surgery, Zurich
- Laboratory for tissue engineering, German Heart Center Berlin, Germany
- Division of Cardiology, University of Zurich
- Division of Radiology, University of Zurich
- NoviNano-institute, Lviv, Ukraine
- Institute of Physics, Humboldt-University Berlin, Germany
- Chair for robotics and Embedded Systems, TU Munich, Germany
- MEDIRA MedTech, Germany
- Telebionics, USA