Thoracic Surgery Research
Head
|
|
|
Prof. Isabelle Schmitt-Opitz, MDDirector Department
|
PD Sven Hillinger, MDCo- Head of Research
|
Organization
Research in thoracic surgery focuses on different areas, such as oncology and transplantation.
Oncology
Malignant Pleural Mesothelioma (MPM)
One of the topics in cancer research is the identification of novel biomarkers and development of therapeutic strategies for malignant pleural mesothelioma (MPM), an incurable thoracic malignancy related to asbestos exposure. To achieve these goals, various tumor tissue biomarkers that can help in the prediction of disease aggressiveness and response to treatment are assessed in translational studies (protein expression, mutation profiles and microRNAs). For example, could we identify that mutations or deletions of the BAP1 gene were associated with resistance to chemotherapy.
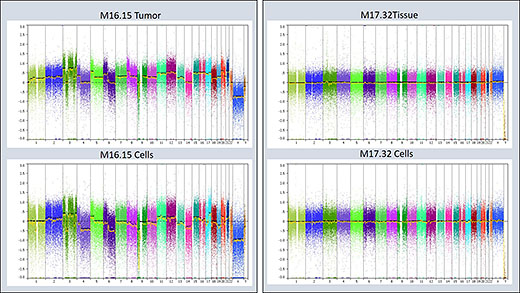
Figure 1: Copy Number Variation (CNV) analysis applied for verification of primary cell lines. Left: Example of a CNV analysis of a primary MPM cell line and the matching primary tumor tissue, in which the observe chromosomal changes are identical. Right: Example of a non-MPM primary cell line and the matching tissue sample, which both do not show any chromosomal alterations.
Further Projects 1
Furthermore, our current projects particularly emphasize the identification of markers that can be detected in the blood of the patients, such as free circulating or microvesicle (exosomes) encapsulated nucleic acids, as these represent a very attractive tool for non-invasive diagnosis, outcome prediction, or disease monitoring. Besides, we are exploring novel targets for the treatment of MPM using in vitro cell models and pre-clinical animal models. For this, we are establishing a primary cell bank for mesothelioma.
In addition, we currently apply intracavitarily cisplatin/fibrin after macroscopic complete resection in a phase II trial to prevent local tumor recurrence (NCT01644994). So far, we included 17 out of 20 patients in the protocol. In parallel, we are evaluating the combination of intracavitary cisplatin/fibrin with post-operative radiotherapy in a preclinical animal model.
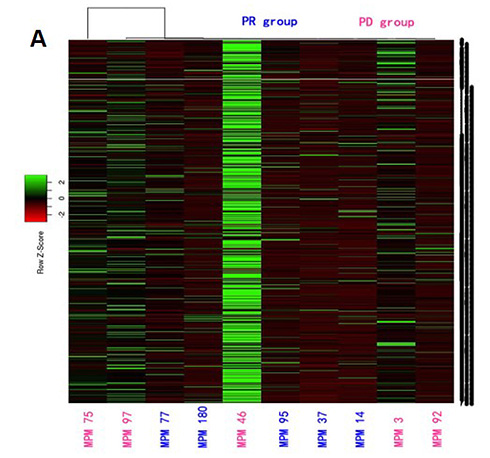
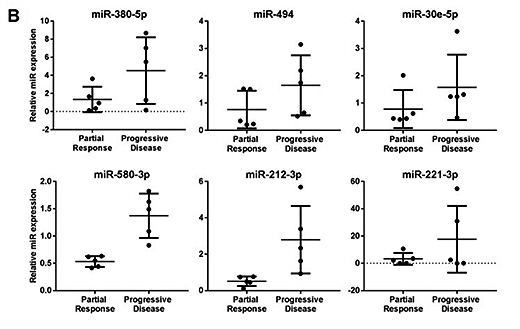
Figure 2: Identification of biomarkers associated with response of MPM to chemotherapy. A) Heat map obtained from profiling of 754 individual microRNAs. B) Expression of selected microRNA biomarekrs candiadtes in tissue samples from responders (partial response) and non-responders (progressive disease) to cisplatin-pemetrexed chemotherapy
Further Projects 2
In further studies aiming to achieve a better understanding of MPM, we used experimental animal models exposed to asbestos to explore mechanisms of mesothelioma development. This led us to the discovery of pathways activated in pre-neoplastic lesions and we are currently characterizing them. We made public our collection of live-biobank of primary mesothelioma cells and we keep charactering them at the genomic level. We also explored how growth in 3D under different stiffness conditions changes the phenotype of mesothelioma cells.
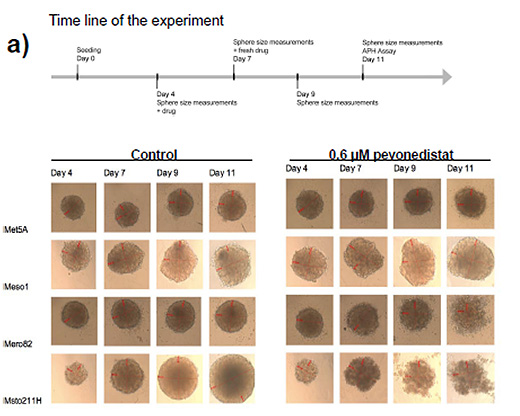
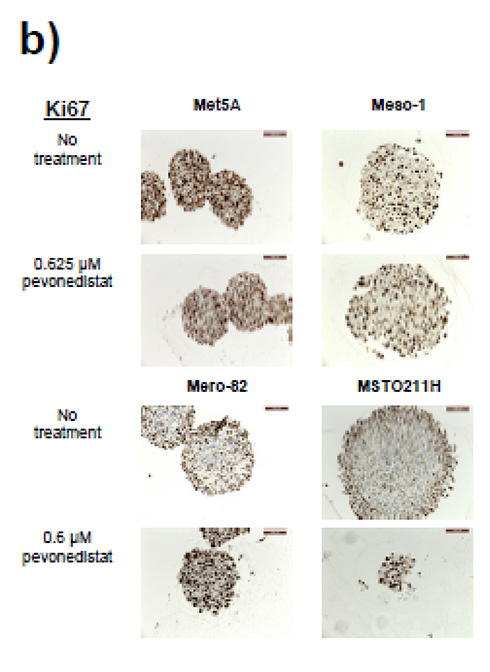
Figure 3: Effect of pevonedistat on CUL4A overexpressing MPM cell lines grown in 3D. a) Cells were grown in 3D spheroids, and 4 days after seeding, spheres were treated with either pevonedistat or DMSO. The presence of dead cells was clearly visible only in CUL4A overexpressing cell lines (Mero82 and MSTO211H). b) The accumulation of cells positive for Ki67 (proliferation markers that are positive in proliferating cells especially in S/G2 phase) were detected in the remaining cells in the spheroids of Mero-82 and MSTO211H.
Lung Cancer
As lung cancer is the most prominent cause of cancer-related death, novel treatment strategies are urgently needed. In our research work, we found CD26/DPP4 to be significantly increased in lung adenocarcinoma compared to normal lung. Moreover, a higher CD26/DPP4 expression was significantly correlated with a worse survival of patients suffering from adenocarcinoma.
On the base of these clinical data, we hypothesized that the inhibition of CD26/DPP4 can suppress lung cancer. In order to test our hypothesis, the CD26/DPP4 inhibitor vildagliptin was applied in a mouse model of lung adenocarcinoma induced by subcutaneous injection of the Lewis Lung Carcinoma (LLC) cell line. We found that vildagliptin treatment significantly reduced the size of tumor via activation of intratumoral NK cells. We therefore deem that CD26-inhibition can be a novel target to treat lung cancer.
In order to validate previously discovered prognostic biomarkers in lung adenocarcinoma (LAC) we are currently developing in collaboration with a group in ETHZ, a mass spectrometry approach called SWATH (Figure SWATH) for the quantitation of serine hydrolases (SH) enzymatic activities. With the newly established ABPP-SWATH MS workflow and starting with 24 LAC stage IIIA biopsies, we obtained MS/MS spectral information. Our ultimate goal is now to measure the proportion of “inactive SHs” from the “total” SHs and potentially follow 400 enzymes activities of the SH superfamily in our lung resection specimens.
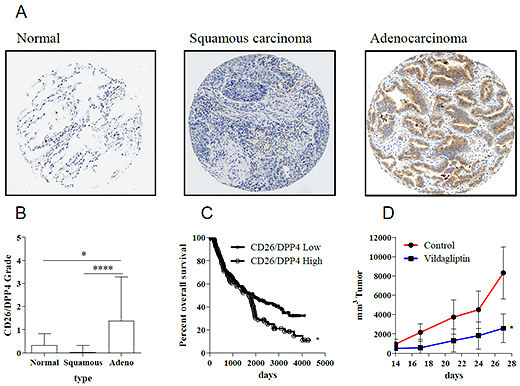
Figure 4: Expression of CD26/DPP4 in lung cancer and potential therapeutic target. CD26/DPP4 stain shows significantly higher expression in adenocarcinoma compared to normal lung or squamous carcinoma (A, B). Overall survival of adenocarcinoma patients is significantly correlated with the expression level of CD26/DPP4 (C). The treatment of CD26/DPP4 inhibitor Vildagliptin suppressed growth of tumor in a model of lung cancer (D).
Lung Transplantation
In order to overcome organ shortage for lung transplantation and reduce waiting list mortality our group is working on ex vivo lung perfusion technology to improve the function of damaged lung grafts. We are doing experiments in large and small animal models with oxygen carrier (perfluorocarbon octyl bromide). Perfusion temperature is also one of the targets in our optimization strategy. We also investigate the potential of adeno-associated virus as vector for gene therapy in a small animal model during ex-vivo lung perfusion in order to reduce ischemia reperfusion injury and the allo-immune response after transplantation.
Collaborations
- Institut klinische Biochemie der Universität Antwerpen, Belgien
- Eidgenössische Technische Hochschule (ETH) Zürich, CH
- Klinik für Pneumologie, Universität Leuven, Belgien
- Institute of Physiology, Perelman University Pennsylvania, Philadelphia, USA
- Institut für Molekularbiologie, Universitätsspital Zürich, Universität Zürich, CH
- Klinik für Immunologie, Universitätsspital Zürich, CH
- Centre Hospitalier, Department of Thoracic Surgery, Strasbourg, France (Gilbert Massard)
- Dr. Shampa Chatterjee, Associate Professor, Institute for Environmental Medicine University of Pennsylvania
- Gilles Willemin, Mouse Metabolic Evaluation Facility (MEF), Center for Integrative Genomics, University of Lausanne
- Dr. Serena Di Palma, Functional Genomics Center Zurich, ETH Zurich/University of Zurich
- Dr. Keke Yu, Department of Pathology, Shanghai Chest Hospital, Shanghai, China
- Dr. Tatjana Sajic and Prof. Ruedi Aebersold, Department of Biology, Institute of Molecular Systems Biology (IMSB), ETH Zurich, Switzerland
- Dr. S. Gray, Translational Cancer Research Group, Trinity Center for Health Sciences, Institute of Molecular Medicine, St. James’s Hospital, Dublin, Ireland
- Prof. Dr. H. Moch, PD Dr. A. Soltermann, Dr. B. Vrugt, Institut für klinische Pathologie, UniversitätsSpital Zürich
- Prof. Dr. M. de Perrot, Dr. G. Allo, Dr. M. Tsao, Dr. Licun Wu, Division of Thoracic Surgery, Toronto General Hospital and Princess Margaret Hospital, University of Toronto, Toronto, Canada
- Dr. V. Serre Beinier, Département de chirurgie, Université de Genève
- Prof. Dr. W. Klepetko, Dr. M. Hoda, Division of Thoracic Surgery, Medical University Vienna
- Prof. Dr. R. Bueno, Department of Surgery, Brigham and Women’s Hospital, Boston
- Dr. A. Jetter, Institut für Pharmakologie und Toxikologie, UniversitätsSpital Zürich
- Prof. Dr. D. Günther, Labor für organische Chemie, ETH Zürich
- Prof. Dr. B. Seifert, Department of Biostatistics, Epidemiology, Biostatistics and Prevention Institute, University of Zürich
- PD Dr. T. Frauenfelder, Dr. D. Nguyen-Kim, PD Dr. A. Boss, Institut für diagnostische und interventionelle Radiolgie, UniversitätsSpital Zürich
- Prof. Dr. M. Pruschy, Fabienne Tschanz, Institut für molekulare Radiologie, UniversitätsSpital Zürich
- Prof. Dr. M. Carbone, Prof. Dr. H. Yang, Prof. Dr. G. Gaudino, University of Hawai’i, Cancer Center, Honolulu
- Prof. Dr. G Reid, Prof. Dr. N. van Zandwijk, Asbestos Diseases Research Institute, Sydney, Australia
- Dr. Alessandra Curioni, Prof. Rolf Stahel, Clinic of Oncology, Zurich University Hospital
- Prof. Maries van den Broek, Institute of Experimental Immunology, University of Zurich, Switzerland
- Dr. Hubert Rehrauer, Functional Genomic Center, University of Zurich
- Prof. Lorenza Penengo, IMCR, University of Zurich
- Prof. Beat Schwaller, Department of Medicine, University of Fribourg
- Prof. Egbert Smit, NKI, Amsterdam
- Dr. Victor Van Beusechem, Department of Medical Oncology VUmc, Amsterdam
- Prof. Dr. Marie Pierre Krafft, CNRS Research Director, University of Strasbourg, Institut Charles Sadron Strasbourg, France
- Dr. Didier Jean and Dr. Marie-Claude Jaurand, INSERM, Inserm U.1162 Research Unit - Universities Paris-Descartes, France
- Dr. Emanuelle Barillot & Dr. Laurence Calzone, Institut Curie, Paris, France

