Reconstruction Research
Head
|
|
|
Prof. Pietro Giovanoli, MDDirector of Reconstructive Surgery |
|
Organization
Research activities in the Plastic Surgery and Hand Surgery lie in the fields of microcirculation, wound healing, connective tissue research, tissue engineering, skin grafts, motion analysis, inflammatory biomarkers in burns, HLA sensitization in burns and vascularized composite allotransplantation.
Skin Tissue Engineering
For 2018, the Microcirculation and Skin Tissue Engineering group lead by Prof. N. Lindenblatt has engaged in several multidisciplinary projects in the fields of wound healing, preclinical drug development and tissue engineering.
Effect of TOP-N53 on angiogenesis and vascular leakage in diabetic mice
Diabetic foot ulcers are a serious complication in diabetic patients, characterized with impaired wound healing and deficient blood supply. In collaboration with Topadur Pharma AG and financed by the Swiss Innovation Agency (Innosuisse), the group is currently performing a preclinical proof-of-concept study in diabetic mice to test the efficacy of TOP-N53, a pro-angiogenic novel compound. The compound can be potentially used to treat diabetic foot ulcers by promoting angiogenesis and oxygen supply.
Molecular profiling of autologous fat graftings
The group is investigating the underlying molecular mechanisms that contribute to the regenerative properties of autologous fat graftings (microfat and nanofat) through mass spectrometry and molecular biology methods. Nanofat is prepared through mechanical shearing and is used successfully in our clinic to treat hyperthrophic scars and rejuvenate skin. The successful identification of factors involved in nanofat’s regenerative properties may lead to advantageous therapeutic benefits, resulting in direct clinical translation and research application in regenerative medicine.
Construct pre-vascularization with adipose tissue
Successful integration of skin grafts and skin substitutes depends on appropriate vascularization. Failed vascularization of grafts causes necrosis and rejections. Adipose tissue is rich in endothelial cells, mesenchymal stem cells and growth factors, which have been reported to significantly enhance biomaterial vascularization. With that, in vivo studies were carried out on C57BL/6 mice in order to investigate the neovascularization process of constructs composed of lipofragments.
In situ bioengineered dressing for chronic wounds
The Lindenblatt group is highly involved in the Hochschulmedizin Zurich Flagship 2016 Project "Skintegrity", where innovative approaches for diagnosis and therapy of skin diseases and of wound healing are being investigated. In collaboration with Prof. S. Ferguson and Prof. K. Würtz (ETHZ), the group is developing a personalized wound dressing that combines electrospun membranes and nanofat as a healing factor.
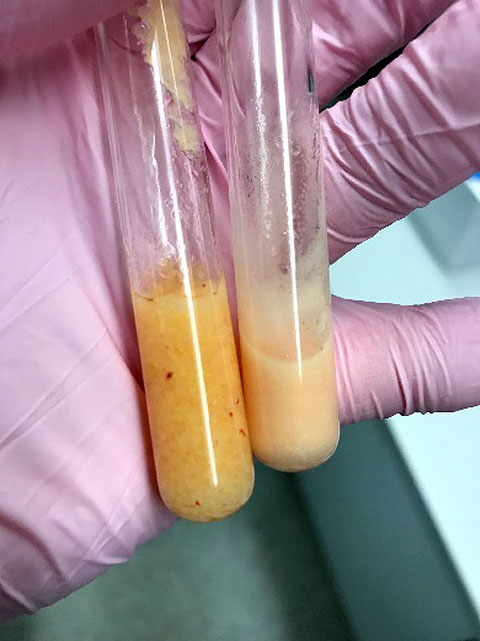
Figure 1: Comparison between microfat (left) and nanofat (right). Nanofat, an oily injectable emulsion,
is prepared through mechanical shearing and filtering of microfat.
Motion analysis
The group of Prof. Dr. med. Maurizio Calcagni performs motion analysis, among others. The aim of motion analysis is to quantify the function of the hand after medical treatment and to determine the effects of restrictions on daily activities. After validation of the method in healthy volunteers, the clinical measurements of 10 patients after partial wrist fusion were completed in 2018. Our results suggest that measurements of the range of motion in the isolated planes of motion (flexion/extension or radial/ulnar deviation) cannot predict the effect of motion restriction on performance in combined movements (such as daily activities). Therefore, postoperative function of the wrist could be better assessed with measurements of wrist mobility in combined planes, in addition to the classical goniometer measurements. In order to transfer these results into clinical practice and to ensure easy application, we are currently working on the validation of a portable sensor for the wrist. Furthermore, the application of our three-dimensional motion analysis routine will continue in 2019 for patients with major injuries of the fingers.

Figure 2: Motion analysis laboratory, equipped with a set of cameras to assess finger movements during daily acitvities.
Epidermal Autografts
A further project is to optimize the production of cultivated epidermal autografts (CEA) and therewith the quality as well as the safety of the CEA for the treatment of severe burn patients.
The team of PD Dr. Maurizio Calcagni has discovered different biomarkers to screen the keratinocyte sheets for safety and efficacy at various stages of their development. Furthermore, we were able to show that the influence of temperature, specifically that hypothermal conditions alter and even enhance secretome release of human keratinocytes in vitro. We could demonstrate, that intermediate cultivation temperature of 33°C beneficially affects the production of immunomodulatory (IFN-γ, GM-CSF), anti-inflammatory (IL-10, IL-4) as well as angiogenic (VEGF, PDGF-bb) biofactors released by keratinocytes during CEA cultivation. In fact, pro-inflammatory factors (IL-1b, IL-8, TNF-α) are also most expressed in the intermediate temperature range; however, they show a positive feedback loop in wound healing.
These findings could improve the overall CEA production (Figure 3). The proteomic analysis could serve to identify a maximal engraftment window and therewith may provide better clinical results with enhanced graft take rate in patients.
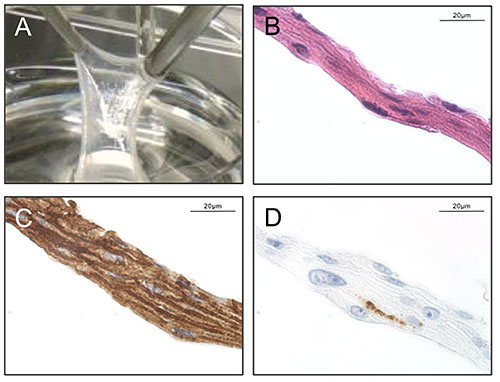
Fig 3a:The cultivated epidermal autografts (CEA) harvested after 21 days (A)show a cell multilayer (B, Hematoxylin Eosin) of keratinocytes in an early stage differentiation status (C, Desmoglein 3). In contrast the late stage differentiation is only slightly detectable (D; Filaggrin). (Magn. x63)
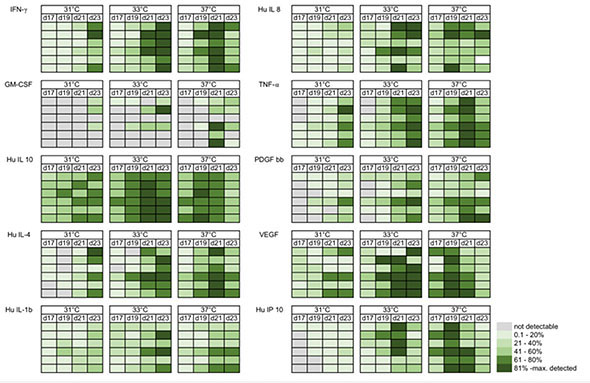
Fig 3b: Heat map indicating the time and temperature dependent cytokine release from human keratinocytes during CEA cultivation in vitro
Burns and Reconstructive Surgery
The group of Prof. Dr. Jan Plock is focusing on clinical research in burns and reconstructive surgery and vascularized composite allotransplantation in basic science.
Clinical Research
Inflammatory biomarkers in burns
Pancreatic stone protein (PSP) has recently emerged as promising diagnostic and prognostic marker in severe trauma. Preliminary analyses from our group suggested serum PSP levels to be strongly associated with the presence of infection and mortality irrespective of the patients’ age and total burned surface area. In that way, PSP might serve as helpful biomarker for timely identification of patients needing intensified medical care. So far, we have included over 100 patients giving rise to a comprehensive data-/biobank. As to that, future analyses will also address further inflammatory biomarkers in burns beyond PSP.
HLA sensitization in burns
Since the inception of clinical VCA over a decade ago, burn victims have been identified as immunologically complex patients for these procedures due to preformed (HLA) antibodies. Yet it remains unclear whether detected HLA antibodies are the result of former alloantigenic events or if their de novo formation occurs during primary burn care. Our interim analysis demonstrated that the formation of HLA antibodies during acute burn care might be lower than previously expected by using glycerol-preserved donor skin as well as restrictive administration of blood products as only 3 patients demonstrated verified de novo sensitization during acute burn care.
Multicenter Studies
In collaboration with Children’s Hospital skin tissue engineering group (Profs. Meuli, Reichmann, Schiestl) clinical studies regarding autologous tissue engineered skin replacement have been initiated (TBRU-ds-BA-PIIb and TBRU-ds-RAC_PII). Moreover funding from the Swiss National Science Foundation was obtained for developing the clinical aspects of skin tissue engineering further (IICT 180418).
Together with Prof. Annelies Zinkernagel and PD Dr. Barbara Hasse from the Department of Infectious Diseases we were able to collaborate for a Clinical research priority program funded by the University of Zurich and by the Swiss National Science foundation (BacVivo – Precision medicine for bacterial infections; SNSF 31BL30_185401).
Basic Science – Vascularized Composite Allotransplantation
Graft Vasculopathy
Graft vasculopathy is a characteristic feature of acute and chronic rejection, that are both inevitable during allotransplantation. To better understand the pathophysiology of graft vasculopathy, this project studies inflammation and extracellular matrix remodeling within the vascular wall. The study is based on a rat model of hind limb transplantation and an in vitro model of vasculopathy.
Immunomodulation
To reduce the burden of immunosuppression in allotransplantation the potential of mesenchymal stromal cells (MSCs) is explored. While prolongation of graft survival has been achieved, long-term survival through immunomodulation and Treg upregulation rather than a tolerance approach is currently our goal. In a translational approach we examine combinations of systemic immunosupression as well as regional modulation of the regional allorecognition process. Furthermore MSCs interfere with the development of graft vasculopathy. The therapeutic or prophylactic potential is further investigated.
Connective Tissue Research and Tissue Engineering
In collaboration with the ETH Zurich (Prof. Vogel) and the company ab medica, Italy, tendon rupture repair by an implant has been optimized: an emulsion electrospun and a coaxially electrospun DegraPol® tube that is biocompatible, biodegradable and very elastic, enabling the surgeon an easy handling during implantation has been developed and tested in the full transection rabbit Achilles tendon model. Biomechanical results are promising, because three weeks post-operation, tensile strength was doubled if a growth factor was applied compared to the factor-free situation. Moreover, in-depth histological analyses were performed.
Bone tissue engineering in vitro is performed and several new approaches including bioreactors are conducted (collaboration with Prof. Stark ETH Zürich). The impact of calcium phosphate nanoparticles was investigated in terms of gene expression in human adipose-derived stem cells, aiming at a spontaneous osteogenic differentiation without medium supplementation.
A novel method to assess perfusion capacity by MRI and vascular architecture and morphology by micro-CT in different biomaterials intended at bone regeneration is being developed (collaboration with Prof. Emmert (Wyss Translational Center Zurich and Charité Berlin). For that purpose, biomaterials are grown on the chorioallantoic membrane of the chicken embryo and after one week analyzed by MRI in vivo and by micro-CT after automated perfusion with MicroFil® ex vivo.
Collaborations
-
Dr. Aldo Ferrari, PhD, Dr. Simone Bottan, PhD. Hylomorph AG and Laboratory of Thermodynamics in Emergin Technologies. ETH Zurich
-
Prof. Dr. Sabine Werner, Laboratory of Tissue Repair and Cancer, ETH Zurich
-
Topadur Pharma AG, Schlieren
-
Ast. Prof. Dr. Tomás Egaña, PhD, Pontificia Universidad Católica de Chile, Santiago, Chile, and TUM Munich, Germany
-
Prof. Dr. Brigitte Vollmar, MD, Institute for Experimental Surgery, University of Rostock, Germany
-
Prof. V. Vogel, PhD, ETH Zurich
-
Prof. W.J. Stark, PhD, ETH Zurich
