Animal Welfare in Biomedical Research
Pain Management
An important reason for suffering in experimental animals is pain induced by invasive procedures, diseases and injuries. But pain management is more than an animal welfare concern, as it has important scientific and methodological implications for the design of experiments and the quality of the resulting data.
To ensure high-quality scientific outcomes and humane treatment of laboratory animals sufficient anesthesia, reliable alleviation of pain and supporting experimental housing conditions are essential.
In the past, we have developed and evaluated physiology and behavior based pain assessment tools for laboratory mice. Since 2017 we focus our work also on the development of tools for the legally required severity assessment in animal experimentation. Therefore, we combine well-established severity assessment methods with new approaches like cognitive bias testing to detect changes in the animal’s affective state. This work is conducted as part of the research consortium DFG FOR2591 in collaboration with 15 different research groups in Germany and Switzerland. Within the consortium we are collaborating with several research groups to establish remote and automated systems to grade severity fast, objectively and in a non-invasive way (e.g. Automated Mouse Grimace Scale). The overall aim is the assessment of severity in different animal models with validated and easy-to-use parameters. With the implementation and evaluation of refinement measures (e.g. improved housing conditions or local anesthesia) we aim to minimize severity and to contribute to the 3R principles.
These tools are also used to improve analgesia protocols for the treatment of pain in mice without affecting experimental read-out. We characterized for example the opioid Tramadol (Fig. 1) as well as Tramadol-Paracetamol combinations in abdominal surgery (Fig. 2) and in orthopedic models and the antipyretic Paracetamol in embryo transfer surgery. In a collaboration with the University of Basel we are currently developing a new sustained release buprenorphine formulation for stress-free and continuous analgesia administration in rodents.
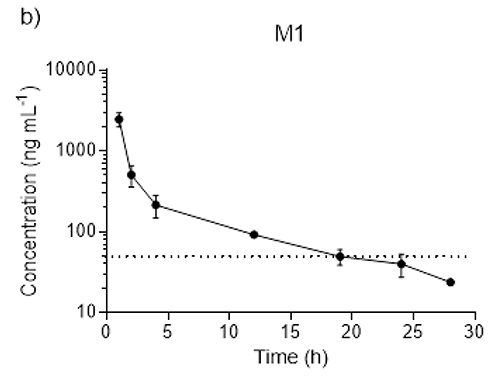
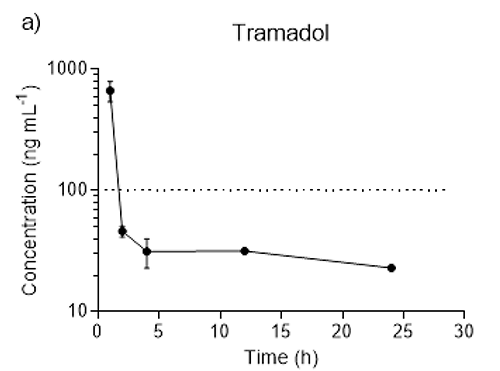
Figure 1: Serum concentrations after oral Tramadol administration.
a) Semi-log plots of mean serum tramadol concentrations over time (h) after subcutaneous administration of 25 mg kg-1 tramadol followed by 25 mg/kg of tramadol in drinking water for 24 hours. The horizontal line represents the minimal analgesic concentration of tramadol (100 ng mL-1) in humans.
b) Semi-log plots of mean serum M1 concentrations over time (h) after subcutaneous administration of 25 mg kg-1 tramadol followed by 25 mg kg-1 of tramadol in drinking water for 24 h. The horizontal line represents the minimal analgesic concentration of M1 (40 ng mL-1) in humans.
Reference: Evangelista R, Bergadano A, Arras M, Jirkof P (2018) Evaluation of the analgesic efficacy of a 25 mg/kg Tramadol s.c. injection-oral combination in a surgical model in C57BL/6J mice. JAALAS, accepted.
Burrowing Test
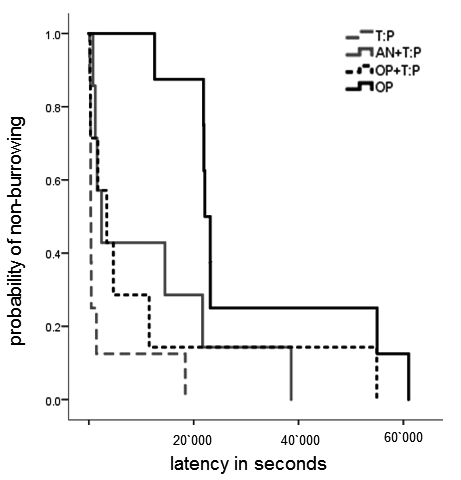
Figure 2: A prolongation of burrowing latency indicates post-surgical pain in mice. Oral Tramadol:Paracetamol treatment results in comparable burrowing latencies as control treatments. Burrowing latencies differed significantly between T:P and An + T:P (p= 0.024) and OP compared to all other groups: OP vs T:P (p < 0.0001), OP vs. An + T:P (p= 0.032) and OP vs. OP +T:P (p=0.019). T:P = TP in drinking water only, An +T:P = anesthesia and T:P in the drinking water, OP+ T:P =anesthesia and surgery with T:P in the drinking water; OP =anesthesia and surgery only.
Reference: Jirkof P, Arras M, Cesarovic N (2018)Tramadol:Paracetamol in drinking water for treatment of post-surgical pain in laboratory mice. Applied Animal Behaviour 198: 95-100, doi.org/10.1016/j.applanim.2017.09.021
Collaborations
- Alessandra Bergadano, Comparative Medicine, F. Hoffmann-La Roche
- Andre Bleich, Institute for Laboratory Animal Science, Hannover Medical School
- Brianna Gaskill, Animal Sciences, Purdue University
- Micheal Czaplik, Department of Anesthesiology, RWTH Aachen University
- Jörg Huwyler, Pharmazeutische Technologie, University of Basel
- Annemarie Lang, Clinic for Rheumatology and Clinical Immunology, Charité Berlin, Germany
- Dorit Mehrhof, Institute of Imaging and Computer Vision, RWTH Aachen University
- Heidrun Potschka, Institute for Pharmacology, Toxicology and Pharmacy, Ludwig-Maximilians-University Munich
- Petra Seebeck, Zurich integrative Rodent Physiology, University of Zurich
- Rene Tolba, Institute for Laboratory Animal Science & Experimental Surgery and Central Laboratory for Laboratory Animal Science RWTH Aachen University